

These trials evaluated the efficacy and safety of andexanet alfa in reversing apixaban and rivaroxaban, respectively, in healthy volunteers. Īndexanet alfa received approval as a breakthrough therapy and as an orphan drug based on the results of two phase 3 trials, ANNEXA-A and ANNEXA-R. It does not currently have approval for the reversal of edoxaban, fondaparinux, or low-molecular-weight heparins due to a lack of sufficient data in patients on these agents. Andexanet alfa is the first FDA-approved reversal agent for factor Xa inhibitors. However, emergency room visits and hospital admissions for bleeding due to factor Xa inhibitors have risen with the increasing use of these agents. They are also convenient in that less routine blood monitoring is required than with the use of warfarin. įactor Xa inhibitors have demonstrated effectiveness as warfarin with a better safety profile in terms of bleeding.
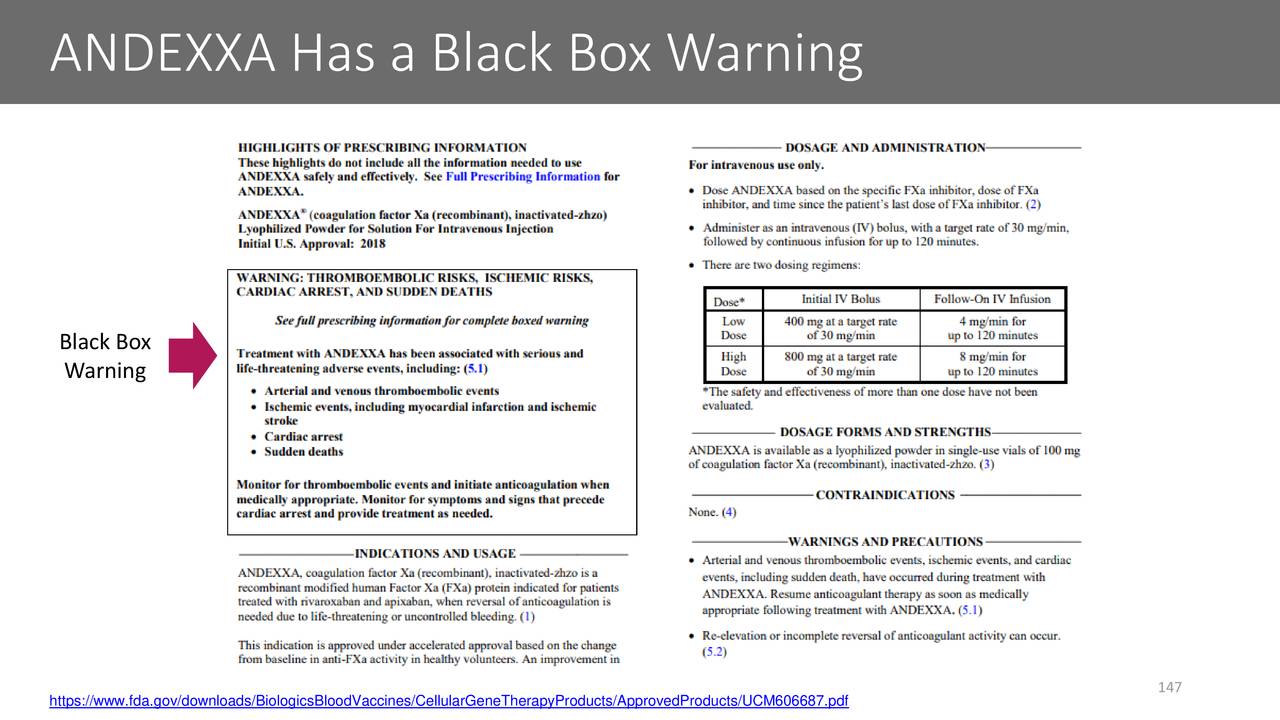
Andexanet alfa is a recombinant modified factor Xa protein approved by the FDA in May 2018 to reverse apixaban and rivaroxaban in patients with life-threatening or uncontrolled bleeding.


 0 kommentar(er)
0 kommentar(er)
